In 2017, nine critical raw materials were added to the EU’s Critical Raw Materials list. While the 27 materials that made the list are all “critical”, we have selected another three of the new additions, which stand out as of particular importance to current and future European societal needs.
Hafnium – high-temperature alloys and ceramics
Hafnium is a hard, ductile metal similar to stainless steel in its appearance and chemically very similar to zirconium. In nature, hafnium is always found with zirconium and its main commercial sources are zircon and baddeleyite; these are available as by-products from the extraction of titanium minerals. Commercial production of hafnium is driven by demand in the nuclear industry for high purity zirconium metal alloys. Major uses of hafnium are super alloys and nuclear reactor control rods. Hafnium is used in high-temperature alloys and ceramics, since some of its compounds are highly refractory with extremely high melting points.
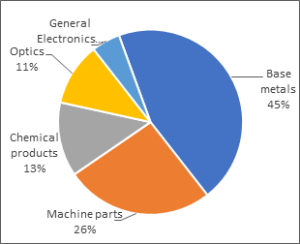
Fig. 1. Global end uses of Hafnium
The main applications for hafnium include:
Super alloys: the major application for hafnium is as an alloy addition in polycrystalline nickel-based super alloys; for example, MAR-M 247 alloy contains 1.5% hafnium. These alloys are used in the aerospace industry, both in turbine blades and vanes, and also in industrial gas turbines. The super-alloy industry requires the purest form of hafnium, crystal bars, with low zirconium content. Demand and supply for this form of hafnium is approximately equal, making the sector volatile.
Nuclear control rods: hafnium and zirconium are both used in nuclear reactors and nuclear submarines. Both hafnium and zirconium must be in the pure form in order to work effectively, this leads to the production of hafnium-free zirconium and, as a result, hafnium as a by-product. Hafnium is used in nuclear control rods due to its high thermal neutron absorption cross section.
Other uses of hafnium are as refractory ceramic materials, in microchips and nozzles for plasma arc cutting.
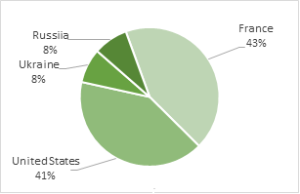
Fig.2. Global Hafnium refinery production. Total annual production is around 72 000 tonnes
Natural Rubber – a biotic raw material
Natural rubber is primarily harvested from the rubber tree Hevea brasiliensis. Although this tree is native to the Amazon region, over 90% of natural rubber is now produced in Southeast Asia. Use of Natural Rubber in European value chains is dominated by the tyre industry, whereas in Asia General Rubber Goods (GRG) applications in high-tech industries play an important role. There are many uncertainties in natural rubber production for both end-user and producer given the biotic nature of the raw material.
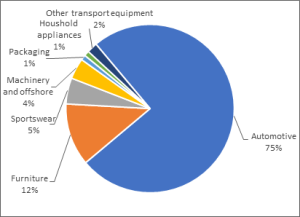
Fig. 3. Global end uses of Natural Rubber
The management of used tyres is well organised in Europe and quite successful. In 2015, 92% of those tyres were either reused as second-hand tyres or reconditioned through retreading, or – when unsuitable for further use on a vehicle – recycled or sent to energy recovery as End-of-Life Tyre (ELT).
The tyre industry uses up to 75% of natural rubber consumed in the EU. An average car tyre will contain 15% natural rubber by weight and a truck tyre will contain, on average, 30%. The remaining content of tyres consists, among others, of synthetic rubber, carbon black and silica as tyre fillers, steel cord and wires to provide strength and other chemicals such as oils and zinc oxide.
Other General Rubber Goods uses can be divided into: Industrial products, such as moulded and extruded products, belting, hose and tube.
Final consumer products, such as footwear, toys, sports and leisure goods; and latex products, such as dipped goods, thread, adhesives, carpet underlay, gloves and condoms.
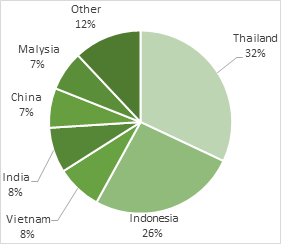
Fig.4. Global production of natural rubber. Total annual production is around 11.7 million tonnes
Scandium – Fuel cell enabler
The name Scandium originates from the Latin ‘Scandia‘ for Scandinavia where it was discovered. It is a silvery-white light transition metal and its main properties are its light-weight (density of 2.99 g/cm3, close to aluminium), high melting point (1 541°C) and small ionic radius.
Scandium is not particularly rare; its abundance in the upper continental crust is 14 ppm. However, due to the small size of its ions, it does not selectively combine with the common ore-forming anions, and rarely forms concentrations higher than 100 ppm in nature. It shares similar characteristics with Rare Earth Elements (REEs) but has quite specific geological and industrial properties which justify a distinct classification.
There is currently no Scandium production in the EU.
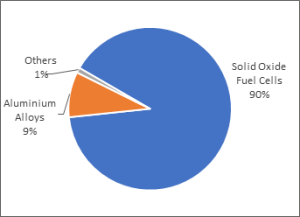
Fig. 5. Global end uses of scandium
The main application for Scandium is in Solid Oxide Fuel Cells (SOFC). In general terms, a fuel cell is an electrochemical cell that converts a fuel source and an oxygen source into an electrical current, plus water, CO2 and heat. It does this by promoting reactions between the fuel and oxidant (reactants), which are triggered by a very high temperature environment (1 000°C). There are a number of types of fuel cells, but SOFC design appears to be the current leader.
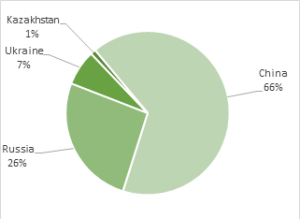
Fig. 6. Global mine production of scandium oxide. Total annual production is around 15 tonnes of scandium oxide
The second most important use is as an alloying element with aluminium. Aluminium-scandium (and magnesium) alloys are some of the lightest alloys known and could help increasing fuel efficiency in aerospace and automotive transportation. Small additions of scandium have interesting effects on aluminium alloys delivering materials with significantly improved properties.